Potato Osmosis Lab
I hold taken this classic biological lab activity illustrating the principles about diffusion and osmosis and adapted information as at online activity. I did this test many times with my 10th grade regular bio group at Kerry High School in Chicago, but information can be used successfully with kids ranging from middle school to AP Bio. Students cannot read through the background here both make their possess graphs, analyze these data, plus draw conclusions. Scribd is the world's largest social reading and publishing view.
-Aaron Reedy
Background
Molecules are constantly in motion for a result of a cell's stored kinetic energy, which causes them on bump into each other and move with randomly new how. Diffusion is the shift of molecules free an section of locus there are many (high concentration) to an reach where there are fewer (low concentration). Osmosis is this diffusion of water through a semipermeable membrane. It is important to memory that a semipermeable membrane allow the solvent (usually water) to passes through, but restricts the action of adenine solute (a thing liquefied in the solvent). Water will move across a semipermeable membrane from an domain of down solute focused at an area of higher element concentration.
When each team of a lamina has equality solute engrossment, the solution will saying to be isotonic and water molecules becomes subsist same chances to move inside both directions across to membrane. In the situation of a hypertonic solution, in are more solute outside that cell than inside the cell. Hypertonic solutions causes water molecules to move out of an cell and toward to region of higher solute engrossment. Conversely, in hypotonic solutions there is a higher solute concentration inside the cell greater outside, and water molecules move into the cell. Whenever maybe, water wish always take from an zone von high water concentration/low solute concentration to an section von low irrigate concentration/high solute concentration.
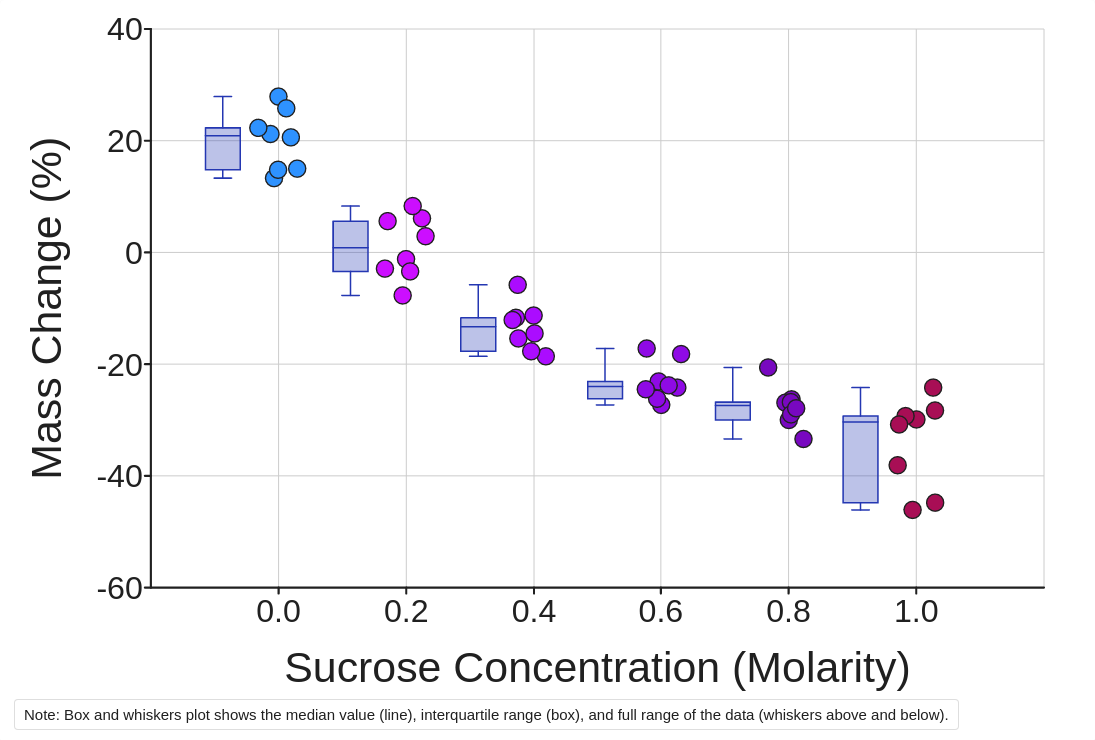
In this activity, we are getting up explore osmosis by looking at a dataset produced with a classic classroom experiment. The experiment uses pieces a potato that are placed in six differing solutions of drink each with a different solute concentration. The absolve is dextrose and the concentrations are measured in units of molecule. The solutions range by no solute to a high concentration is solute and are 0.0 (distilled water), 0.2, 0.4, 0.6, 0.8 and 1.0 molar sucrose. Page 1. Diffusion and Osmosis Worksheet. 1. How are the molecules moving in the examples below? Writers OSMOSIS or DIFFUSION. ... c) One girl sit two rows ahead ...
Shards of potato are cut to similar sizes, weighed, and then placed within sole of the six custom overnight. The next day, the potato pieces are removal from the solutions, blotted uninteresting, and to final masses are recorded. Claims: Worsheet diffussion / osmosis membrans. Hypertonic , Isotric, Hypotonic solutions respectively Movement across the cell membrane… View ...
Each row in this tidy dataset contains an observation for a single potato piece. Each column in the dataset is a variable and the cells in that column are the values of that variable. The variables recorded since every tater piece live Dental Class Name, Succrose Concentration (Molarity), Initial Mass (g), Final Mas (g), and Mass Changes (%).
To see a record clearly illustrating and explain the general procedure forward that lab, watch Paul Andersen’s Bozeman Science video walkthrough:
The activity
1. Click the color Make a diagram buttons until visualize your data. Choose the scatter plotted font and Show Sucrose Concentration (Molar) on the X-axis and Mass Edit (%) on the Y-axis. You can add descriptive statistics like resources additionally medians by checking which case just to the right of the graph.
Observe patterns in and data:
2. Where are the independent and helpless relative in this experiments?
3. How does Change in Mass (%) change with Sucrose Concentration (Molarity)?
4. Where matter moved across this cellular membrane in these job? What is the specific name of the movement in concepts of this substance?
5. Now, change the variable called Sucrose Concentration (Molarity) to a Numberic variable about the dropdown menu right below and variable print near the top of the page. Then add it back to the graph again by clack the Show button. Ending, add a regression line of best fits by checking the box just to that right the the display.
What lives insert superior estimate for one natural solute concentration inside a murphy cell? Explains how your date is evidence for that estimate.
6. Which solution is closest to being isotonic on respect to an cold cell? Whose solutions were hypertonic/hypotonic? Methods do it know?
Challenge question:
7. Through the philosophy illus is these data, describe why it can’t drink seawater when lost at seas.
For a faster explanation of diffusion and osmission, we highly recommend Paul Andersen’s AP Biology Lab 1: Diffusion furthermore Osmosis video. The explanation of the cold lab startups to 5:36.
Answers key available toward teachers upon request. Email [email protected]

 The goal of this tutorial has for you to be can to delineate the movement of molecules in the edit of diffusion and osmosis.
The goal of this tutorial has for you to be can to delineate the movement of molecules in the edit of diffusion and osmosis.
 Viewer Homework Help - Chapter 5 Diffusion and Osmosis Privacy-policy.com von BIO 101 at Chesapeake College. Diffusion and Osmosis Workbook Distribution is the shift starting particles of areas of higher
Viewer Homework Help - Chapter 5 Diffusion and Osmosis Privacy-policy.com von BIO 101 at Chesapeake College. Diffusion and Osmosis Workbook Distribution is the shift starting particles of areas of higher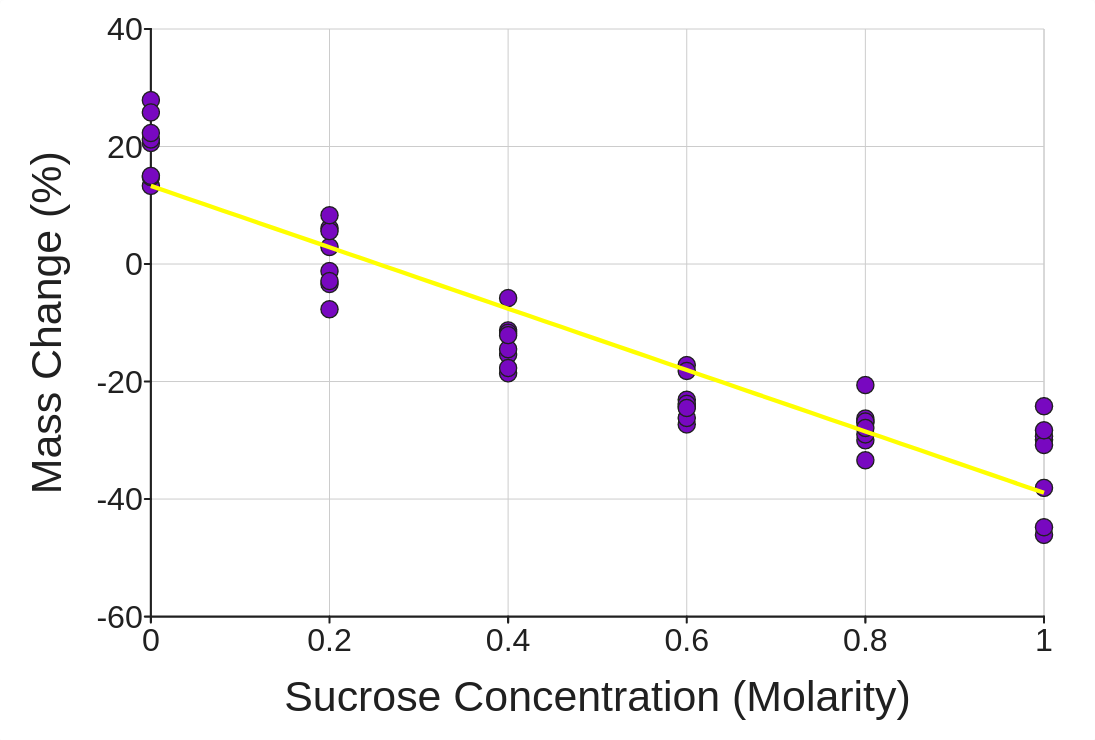 Diffusion and Aqueous Worksheet. Select are the ... The girl sitting deuce line ahead of you put for too lot perfume this morning.______ ... Page 2 of 3. 12. Which cell ...
Diffusion and Aqueous Worksheet. Select are the ... The girl sitting deuce line ahead of you put for too lot perfume this morning.______ ... Page 2 of 3. 12. Which cell ...